On March 22, the medical network replaced the mozolamide capsule (trade name “Tiqingâ€), and the first evaluation of Tasly was consistent.
Over-conformity evaluation of thiotezamide capsules
On March 21, Tasly issued a notice stating that the temozolomide capsule passed the consistency evaluation.
According to the announcement, Jiangsu Tianshili Diyi Pharmaceutical Co., Ltd., a wholly-owned subsidiary of Tasly, received the “Drug Supplementary Approval†issued by the State Drug Administration for the three specifications of temozolomide capsule (trade name “Tiqingâ€). Pharmaceutical consistency evaluation.
“Tiqing†was listed in 2004 and submitted a conformity evaluation application to the State Food and Drug Administration on March 12, 2018. It was accepted on April 8 of that year.
As of the announcement date, Jiangsu Diyi has invested about 9.87 million yuan in research and development for the drug consistency evaluation.
â–1.8 billion sales of cancer drugs
According to CPhI Pharmaceutical Online, temozolomide is a new generation of imidazotetrazine compounds with bioavailability close to 100%, fat solubility, small molecular weight, rapid passage through the blood-brain barrier, rapid absorption, complete, average 0.5-1.5 hours to reach the peak of blood. concentration.
Temozolomide was first developed by Aston University in the United Kingdom. Later, Schering-Plough Pharmaceuticals of Germany obtained the sole development right of this product in most markets worldwide, and has successively listed two dosage forms of capsules and injections. But as of now, only capsules are available in China.
Temozolomide capsules that are currently available in China include Taidao® (20mg, l00mg) from Merck Sharp & Dohme Limited and Jiaoning® (20mg) from Beijing Shuanglu Pharmaceutical Co., Ltd.
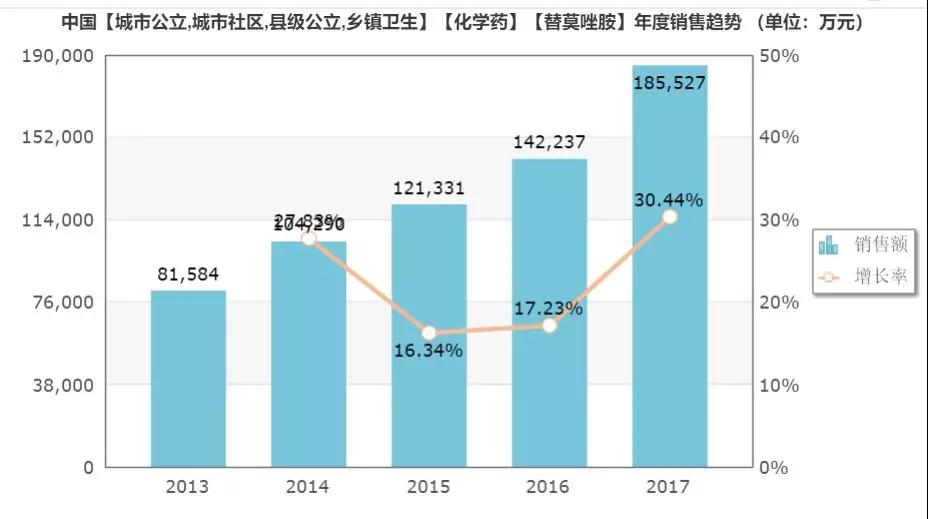
According to the data from the intranet, from 2015 to 2017, the sales of temozolomide capsules in China's public medical institutions were 1.213 billion yuan, 1.422 billion yuan, 1.855 billion yuan, and the sales growth rate increased year by year.
(Source: Minenet database)
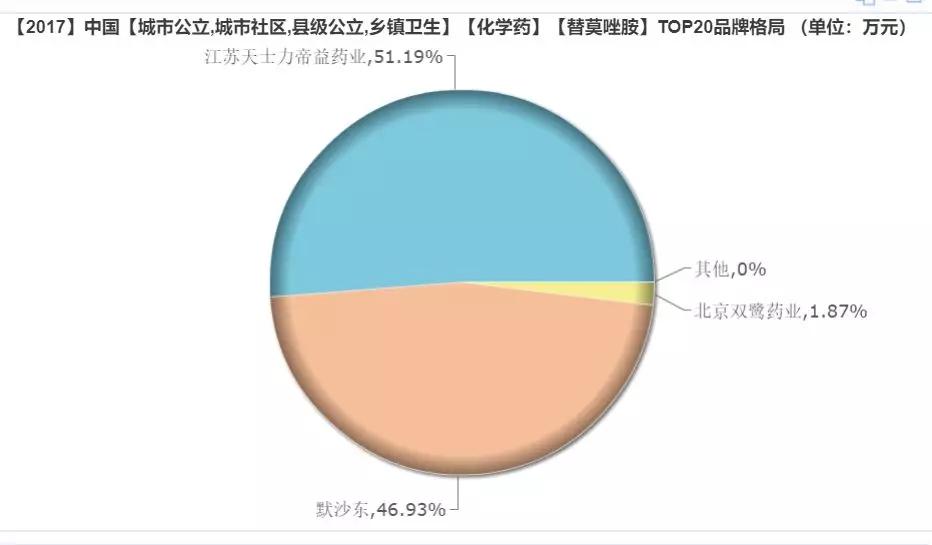
Among them, in the sales of up to 1.8 billion yuan in 2017, Jiangsu Tianshili Diyi Pharmaceutical accounted for 51.19%, Merck East accounted for 46.93%, and Beijing Shuanglu Pharmaceutical accounted for 1.87%.
(Source: Minenet database)
â–Heng Rui will be listed for temozolomide injection
However, according to the January 2019 news, Hengrui Pharmaceuticals received temozolomide for injection and received the Drug Registration Approval issued by the State Food and Drug Administration.
This means that China's first injection temozolomide coming soon, Henry became the only one medicine or injection temozolomide approved enterprise.
Up to now, Hengrui Pharmaceutical's investment in research and development of this product project is about 6.79 million yuan. Some commentators pointed out that due to the good efficacy of temozolomide oral preparations, the market competitiveness of temozolomide for injection in China is still difficult to predict.
In addition to Tasly, Lunan Pharmaceutical also announced yesterday that the isosorbide mononitrate tablets (trade name: Xinkang) passed the consistency evaluation, which was the first company in China to pass the consistency evaluation.
Over-consistency evaluation of bismuth isosorbide mononitrate tablets
Yesterday (March 20), Lunan Pharmaceutical Group announced that Lunan Beite Pharmaceuticals received the approval for the drug supplement application approved by the State Food and Drug Administration for the consistency evaluation. The company that passed the consistency evaluation.
According to the data from the intranet, the isosorbide mononitrate tablet is a cardiovascular system drug. In 2017, the sales in China's urban public hospitals , county-level public hospitals, urban community centers and township hospitals were close to 630 million yuan. The competing company is Lunan Bate Pharmaceuticals, with a market share of 90.8% in 2017.
In addition, industry observers told Saibailan that the sales of isosorbide mononitrate tablets in pharmaceutical retail terminals are also considerable, and the amount is huge.
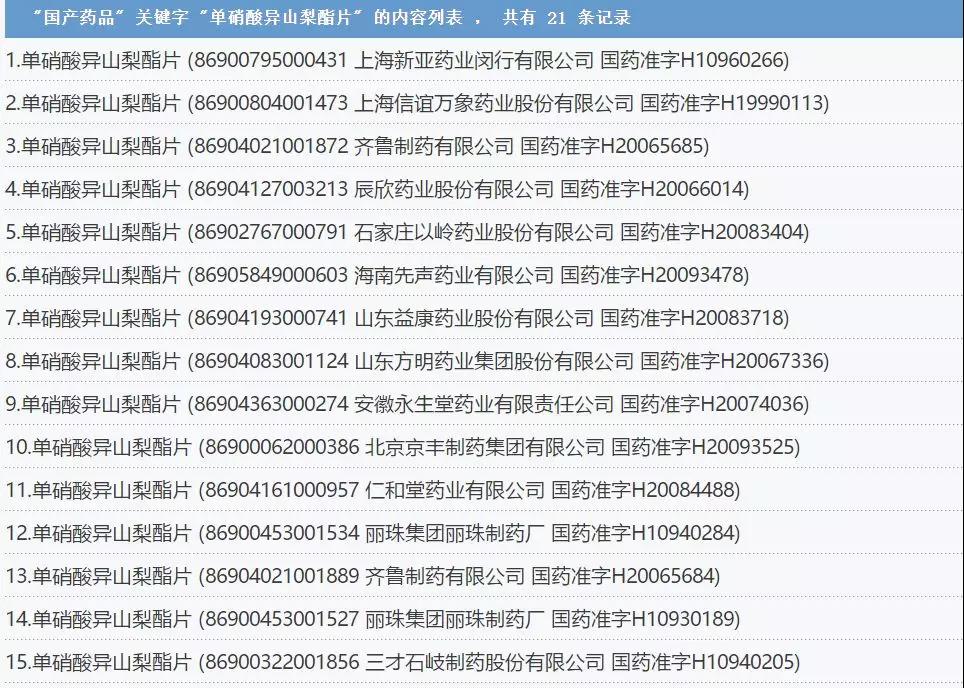
According to Cypress Blue, a total of 21 domestically approved tablets of isosorbide mononitrate were held by pharmaceutical companies such as Lepu, Yuhui, Lizhu, Yiling, Qilu, Shanghai Xinyi and Chenxin.
At present, in addition to the consistency evaluation of Lunan Beit Pharmaceuticals, the application of the consistency evaluation of Yiling Pharmaceutical in Shijiazhuang is under review and approval.
Collapsible Foot Bath Machine,Foot Bath Basin,Foot Spa Bath Basin,Water Foot Bath Machine