On January 23, 2017, Roche Diagnostics announced that its self-developed blood cell analyzer Cobas m 511 has been CE certified and officially launched. This news immediately detonated the IVD circle, which is tantamount to dropping a blockbuster in the field of blood analysis that was originally calm. Roche Diagnostics, a brother of IVD, has a complete product line and a strong competitive advantage. But why has it now had a blood cell analyzer independently developed? What kind of inside story is there behind this?
First, Roche diagnoses the status of rivers and lakes in IVD
First of all, let's take a look at the power map of the IVD world. In the battle for many manufacturers, Roche is the absolute leader who is far ahead, and its status is difficult to shake. Even if Abbott, Siemens and Danaher, among the "Big Four", are making big acquisitions, it seems that they cannot overcome the gap with Roche.
Overview of the IVD industry market and a list of the top 10 companies
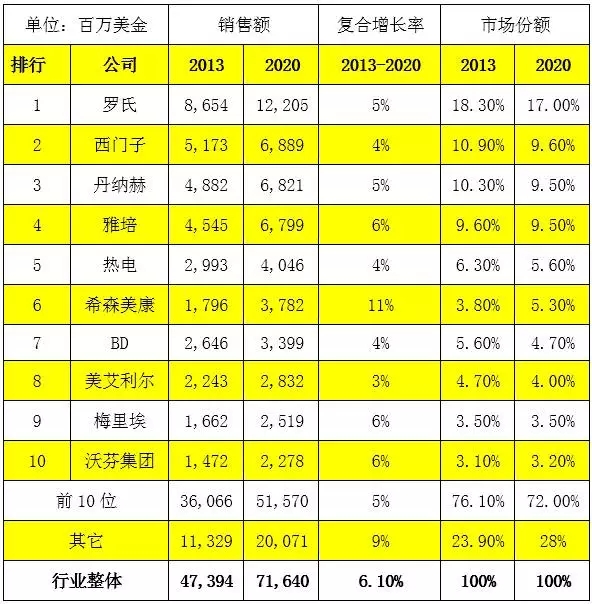
Source: EvaluateMedTech (11 SEP 2014)
Second, the weight of blood analysis in the field of IVD
Second, let's take a look at the weight of the blood analysis field in the entire IVD world. As can be seen from the figure below, compared with the hot chemiluminescence and POCT, the blood analysis is not only the overall market capacity scale (only 5%), but also the annual growth rate is small (2%), and the technology is updated. Particularly slow, it is a typical traditional market. Many people can't understand why, in the face of such a market that is almost "chicken", why should Roche invest heavily again and again?
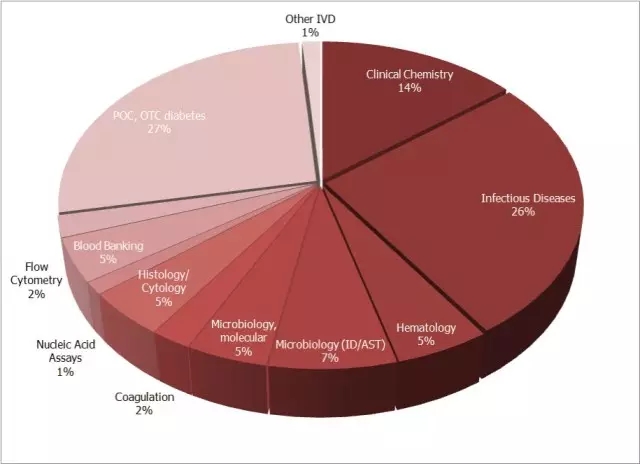
Source: The Worldwide Market for In Vitro Diagnostic Tests, 9th Edition, 2014
Third, the main play of blood analysis
In the segment of blood analysis, where the market is small and the growth is not significant, Roche is indeed difficult. As can be seen from the figure below, the main players in blood analysis are Japan's Sysmex (44.4%), Danaher (Beckman Coulter: 25.4%), and Horiba-ABX (10.7%). ), Abbott (9.8%) and Siemens (9.8%).
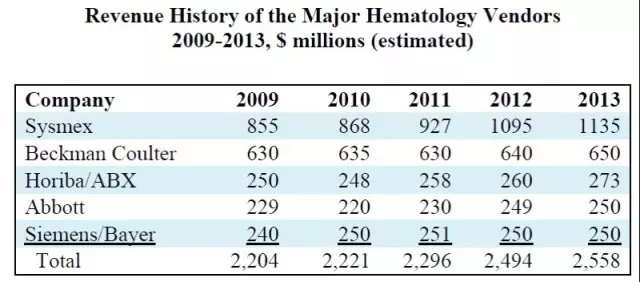
Source: The Worldwide Market for In Vitro Diagnostic Tests, 9th Edition, 2014
Guangdong Widinlsa International Co.Ltd , https://www.guangdongwidinlsa.com