Mary E. Lame, Erin E. Chambers and Kenneth J. Fountain
Waters Corporation (Milford, MA, USA)
Application advantage
■Using CORTECSTM UPLC® columns for narrow peak widths, improved sensitivity and resolution, and matrix interference elimination ■SPE method with Oasis® WCX (a hybrid adsorbent) reduces matrix interference and improves Selectivity of Bradykinin Extraction in Plasma ■Oasis μElution 96-well plate concentrates the sample and minimizes peptide loss, allowing the detection limit of bradykinin in plasma to reach 5 pg/mL
■Highly selective fast SPE extraction (<30 minutes) eliminates time-consuming immunoaffinity purification steps ■Xevo®TQ-S MS achieves high sensitivity in matching with immunoassay methods, but is more complex than the latter Save time and effort ■More accurate and precise than the traditional LBA method ■Fast analysis, only 3.5 minutes, greatly improving work efficiency
Waters solution
ACQUITY UPLC® System
Xevo TQ-S mass spectrometer
CORTECS UPLC C 18 column
Oasis WCX 96-well μElution extraction plate
ACQUITY® 96-well collection plate
Keywords <br> bioassay, Oasis, sample preparation, quantitative peptide, bradykinin, UPLC, CORTECS, plasma
Introduction <br> bradykinin amino acids containing 9 is a physiologically and pharmacologically active peptide, a bradykinin group derived from a protein (FIG. 1). Kinin is an effector that promotes vasodilation, enhanced vascular permeability, nitric oxide release, and arachidonic acid flow. It is an important regulator of blood pressure, kidney function and heart function, and is also involved in the inflammatory response 1 . Bradykinin causes blood vessels to dilate, resulting in a decrease in blood pressure. Therefore, it is extremely advantageous to quantify this hormone peptide with high sensitivity, high selectivity and high accuracy, and to use it as an indicator for disease progression or drug treatment. Although biological agents have previously been quantified by ligand binding assays (LBAs), the use of LC-MS/MS for the analysis of macromolecules has been a trend in the past few years. This is partly because LBAs produce significant cross-reaction problems and lack standardization. LC-MS/MS offers several advantages, such as shorter development times, higher accuracy and precision, reproducible injections, and easy identification of analogs, metabolites, or endogenous interferences with high similarity. Common peptides are often difficult to analyze by LC-MS/MS because they are less susceptible to ionization and are less prone to produce ideal fragment ions, resulting in lower MS sensitivity, making LC and sample preparation methods very difficult to develop. Since the concentration of bradykinin in plasma is as low as pg/mL and the metabolism is fast, it can be artificially produced by proteolysis during blood collection and sample preparation, so accurate quantification is quite difficult.
This study used a specially designed blood collection technique to prevent the formation of bradykinin in vitro, and the use of mixed solid phase extraction (SPE) and high-efficiency solid core particle columns to minimize and eliminate matrix interference while improving quantitative analysis. Sensitivity.
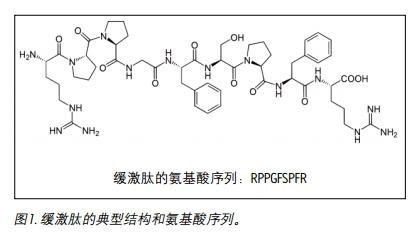
Introduction
Methods conditions <br> system: ACQUITY UPLC
Column: CORTECS UPLC C 18 1.6 μm, 2.1×50mm
Mobile phase A: 0.1% formic acid aqueous solution mobile phase B: 0.1% formic acid acetonitrile solution gradient: see Table 1
Column temperature: 35 degrees Celsius Sample temperature: 15 degrees Celsius Injection volume: 10 μL
Total running time: 3.5 min
Collection plate: Waters 1 mL ACQUITY collection plate
MS condition
MS system: Xevo TQ-S
Ionization mode: ESI+
Capillary voltage: 3.0 kV
Desolvation gas temperature: 500 ° C cone gas flow rate: 150 L / h
Desolvent gas flow rate: 1000 L/h
Collision chamber pressure: 3.58×10(-3) mbar
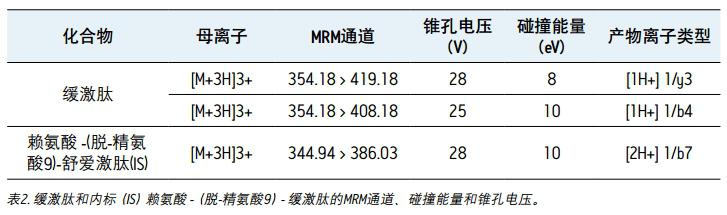
Collision energy: optimized by component, see Table 2
Cone hole voltage: optimized by composition, see Table 2
<br> chromatography data management software: UNIFI®1.6
Quantitative software: UNIFI 1.6
Data Management <br> sample preparation
10 μL of internal standard (IS) lysine-(de-arginine 9)-bradykinin (10 ng/mL) was added to 200 μL of human plasma and mixed. The samples were then 1:1 diluted and mixed using a 5% NH4OH aqueous solution.
The pretreated plasma samples were extracted according to the protocol shown in Figure 2 using the Oasis WCX extraction sample. All solutions are by volume. The entire extraction step was performed on the wells on which the sample was placed on the μElution plate.
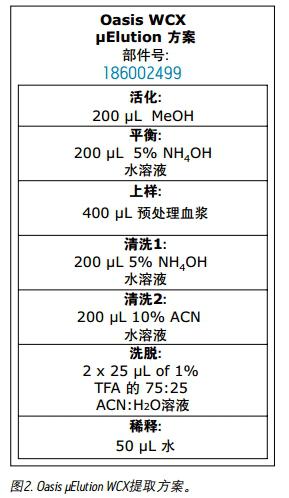
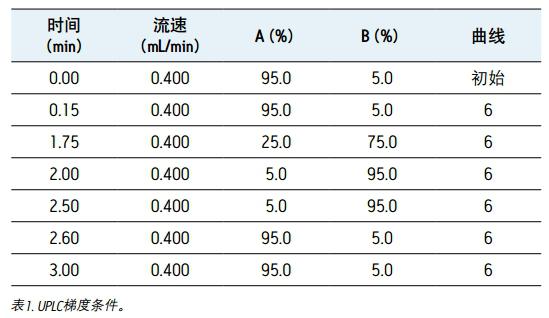
Results and discussion
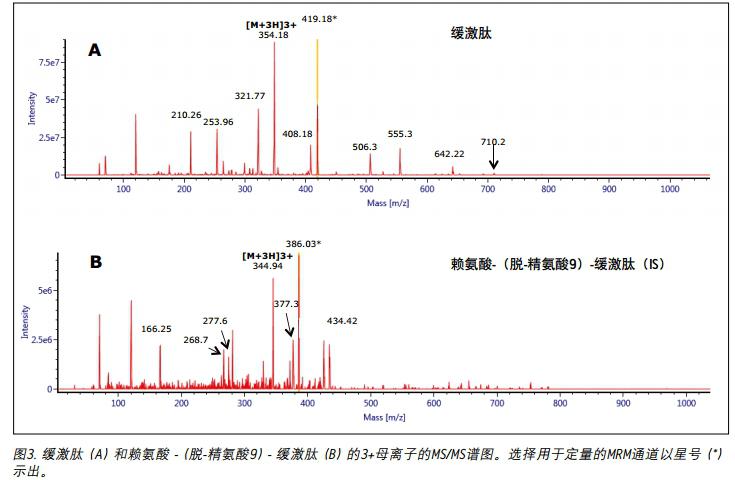
Mass spectrometry The 2+ and 3+ parent ions of bradykinin were observed at m/z 531 and m/z 354. A typical MS/MS spectrum of the 3+ parent ion of bradykinin (panel A) and IS (panel B) is shown in Figure 3. During the method development process, a number of different multi-charged parent ions and fragment ions were recorded. The 3+ parent ions of bradykinin and IS were eventually used because they have the highest strength and selectivity in the matrix. The fragment ions of m/z 419.18y31+ were selected as the main fragment ions for the quantitative analysis of bradykinin. 3+ parent ions and m/z 408.18 b41+ fragment ions were selected for confirmation. For IS, selected m / 3+ and parent ion of m z 344.94 / z 386.03 b72 + fragment ions. The best MS conditions are shown in Figure 2. Although many peptides can generate stronger fragment ions less than m/z 200, these ions (usually imine ions) in the extracted sample can cause high background noise due to lack of specificity. In this assay, the use of highly specific b or y fragment ions with m/z values ​​greater than their parent ions significantly increases specificity, facilitating the use of simpler LC and SPE methods.
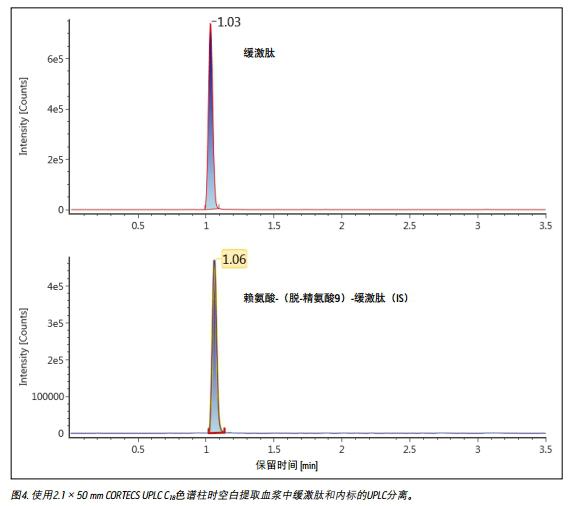
UPLC separation
Unlike small molecules, peptides have poor mass transfer properties in fully porous particles. Therefore, at higher flow rates commonly used in bioanalytical studies, columns filled with solid core particles can achieve sharper peak shapes 2,3 . Compared to fully porous particle columns, when using a CORTECS UPLC C 18 column, bradykinin results in narrower peak widths, reduced tailing, higher peak heights and larger peak areas. The peak width of bradykinin and IS obtained using a CORTECS UPLC C 18 column was 2.5-3.0 seconds, while the peak width was 4 seconds in a fully porous column. A typical chromatogram of bradykinin and IS obtained using a CORTECS UPLC C 18 column is shown in Figure 4.
Sample Preparation
Human plasma was collected into tubes containing EDTA and protease inhibitors to prevent the formation of bradykinin in vitro. Oasis WCX (a hybrid adsorbent) was used for SPE extraction to increase extraction selectivity. This adsorbent relies on reverse phase and ion exchange retention mechanisms to selectively separate bradykinin from complex plasma samples from other high abundance polypeptides. The Oasis μElution 96-well extraction plate enriches the sample without evaporation and reconstitution. This not only saves time, but also reduces peptide loss due to adsorption of the collecting plate wall during evaporation. Optimization of the pretreatment and washing steps is critical for complete recovery of bradykinin in sample pretreatment and SPE extraction. Pretreatment of the sample with an acid or base reduces viscosity and increases contact time with the adsorbent, facilitates inhibition of protein binding, and allows peptides to be better retained in SPE equipment through ion exchange. In the initial method development, a recovery of about 80% was obtained by pre-treating plasma with acid. Since bradykinin is a basic polar peptide with a PI value of 12.0 and an HPLC index of 47.8, it is suspected that it partially eluted during the initial loading step. When pretreatment with a base is carried out, the recovery is increased to about 90%, which is advantageous for improving the initial retention property of bradykinin by ion exchange in the loading step. The acetonitrile solution in the cleaning step 2 was changed from 20% to 10%, eliminating the penetration of bradykinin during the cleaning process and increasing the recovery rate to 100%. The optimized SPE protocol for alkaline pretreatment of plasma prior to loading combined with an optimized wash step with 10% acetonitrile solution achieved complete recovery of bradykinin with a matrix effect of less than 10%. Furthermore, since UPLC separation is performed in reverse phase chromatography, peptide separation by ion exchange imparts orthogonality to the entire method.
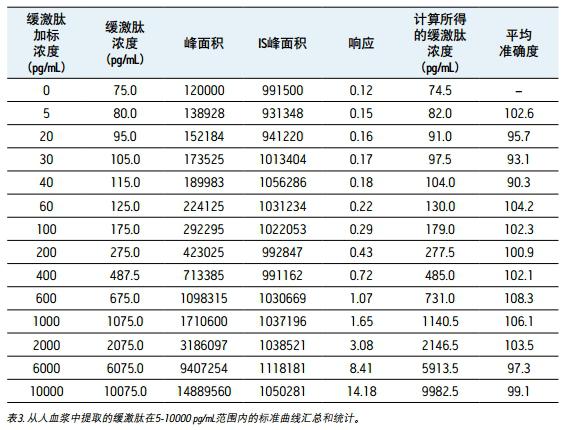
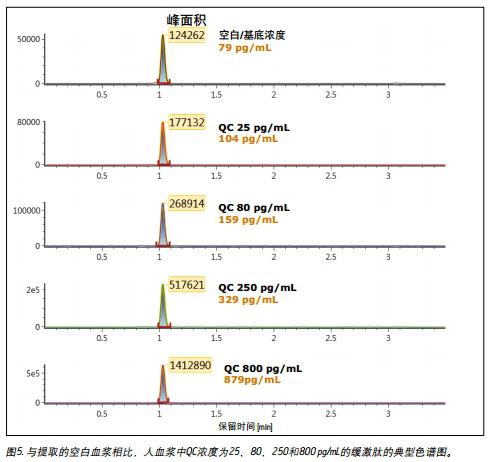
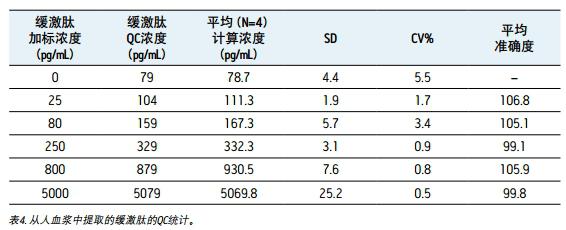
Linearity, accuracy and precision <br> to generate a standard curve, the following final concentrations of bradykinin intensive human plasma: and 5,20,30,60,100,200,400,600,1000,2000,6000 10,000 pg/mL. The following concentrations of quality control (QC) samples were prepared in human plasma: 25, 80, 250, 800 and 5000 pg/mL. Lysine-(de-arginine 9)-bradykinin (final concentration 0.5 ng/mL) was used as an internal standard (IS) for bradykinin. Calculate the peak area ratio (PAR) between the peak area of ​​the analyte and the IS peak. Calibration curves for human plasma samples were constructed using PAR calibration samples and applying a single concentration (1/x) weighted linear regression model. The concentration of all QC samples was then calculated from the PAR values ​​based on the calibration curve. Due to the presence of endogenous bradykinin, standard addition was used. The average basal concentration of bradykinin in the control plasma samples was determined by calculating the x-axis intercept. Then, the substrate concentration of bradykinin was added to all the concentration of the standard and the concentration of QC to achieve accurate quantification. Using a 1/x regression, the curve obtained by bradykinin was linear with an R 2 value > 0.99. Table 3 shows a summary of the performance of the standard curve. The results of QC analysis are shown in Table 4, and a typical chromatogram of endogenous bradykinin and QC is shown in Fig. 5. All concentrations of QC samples showed excellent accuracy and precision, meeting the FDA acceptable standards recommended in the White Paper on Bioanalytical Method Validation White Paper for LC-MS/MS Detection 4,5 . The mean endogenous basal concentration of bradykinin in human plasma was determined to be 79 pg/mL with SD and CV% of 4.4 and 5.5, respectively. The above data confirms that this is a stable and reproducible method.
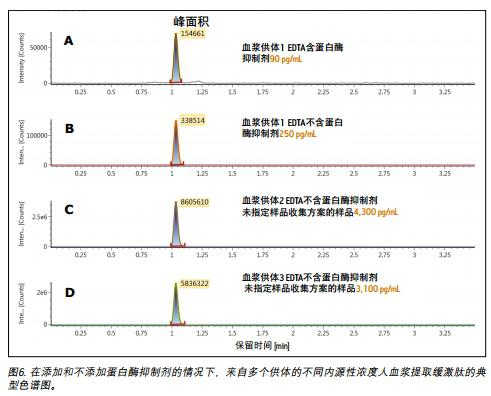
The importance of pre-analysis collection and processing
Accurate quantification of bradykinin in plasma is difficult because bradykinin can be proteolytically produced in blood collection and sample preparation to produce 1,6,7 . This study focused specifically on blood collection protocols to prevent the formation of bradykinin in vitro by the addition of protease inhibitors. In Figure 6, panels A and B show that if the same donor and the same collection protocol were used, blood was collected into EDTA tubes with and without protease inhibitors, and the concentration of bradykinin was from 90 pg/ The mL was raised to 250 pg/mL. The artificial formation of bradykinin was further confirmed by using an unspecified sample collection protocol from two other donors from the supplier, as shown in Figure 6 (Figures C and D). In the above case, blood samples were collected into EDTA tubes without protease inhibitors at concentrations up to a high ng/mL level. These results further demonstrate the need for proper sample collection for accurate quantification of endogenous bradykinin plasma concentrations.
in conclusion
This study developed a mixed-mode SPE extraction method for the extraction of bradykinin in human plasma. The μElution SPE extraction plate eliminates the need for evaporation operations and greatly reduces the potential for loss by utilizing non-specific binding and sample concentration. The bradykinin was separated from the highly similar endogenous interference by a fast, simple, analytical-grade LC method with a total LC run time of only 3.5 minutes. Compared to traditional C 18 fully porous columns, the CORTECS UPLC C 18 Solid Core Particle column improves peak shape and improves sensitivity for bradykinin detection. The combination of CORTECS UPLC C 18 column, mixed weak cation exchange SPE and high m/z b or y MS fragment ions provides ideal levels of selectivity and sensitivity for accurate quantitation and from 200 μL plasma Distinguish the subtle differences in bradykinin concentrations. The standard curve is accurate and precise in the range of 5-100 pg/mL. The minimum concentration of bradykinin that can be accurately quantified above the substrate concentration is 5 pg/mL. All concentrations of QC samples met FDA regulatory standards 4 , 5 with an average accuracy range of 99.8-106.8 and an average CV% of 0.5-5.5. The above data confirms that this is an accurate and reproducible method. This study also confirmed the importance of using appropriate protease inhibitor sample collection methods for accurate determination of endogenous concentrations of bradykinin. Furthermore, if further validation is required, the method also presents great potential for high sensitivity bradykinin quantification of PK and clinically studied patient samples by LC-MS/MS.
references
1. Murphey LJ, Hachey DL, JA. Oates JA, Morrow JD, and Brown NJ. Metabolism of Bradykinin In Vivo in Humans: Identification of BK1-5 as a Stable Plasma Peptide Metabolite J Pharmacol Exp T her July 1, 2000 294: 263-269
2. Wagner BM, Schuster SA, Boyes BE, Kirkland JJ. Superficially porous silica particles with wide pores for biomacromolecular separations. Journal of Chromatography A. 2012; 1264(0): 22-30
3. Kirkland JJ, Schuster SA, Johnson WL, boyes BE. Fused-Core Particle Technology in High-Performance Liquid Chromatography; An Overview. Journal of Pharmaceutical Analysis.
4. Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R, Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays, Pharm. Res., 24 (2007) 1962-1973.
5. Bansal S, DeStefano A, Key elements of bioanalytical method validation for small molecules, AAPS J., 9 (2007) E109-114.
6. Cugno M, Agostoni P, Brunner HR, Gardinali M, Agostoni A, Nussberger. Plasma bradykinin levels in human chronic congestive heart failure J. Clin Sci. (Lond). 2000 Nov; 99(5): 461-6.
7. Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio-oedema. Lancet. 1998 Jun 6; 351(9117): 1693-7.
Acknowledgements <br>Thanks to Jeffrey Widdos and Biological Specialty Corporation for their services and assistance in the collection of human plasma for this study.
PVC Suction Catheter,High Quality PVC Suction Catheter,PVC Suction Catheter Details
K-MED Co., Ltd. , https://www.kmedasia.com