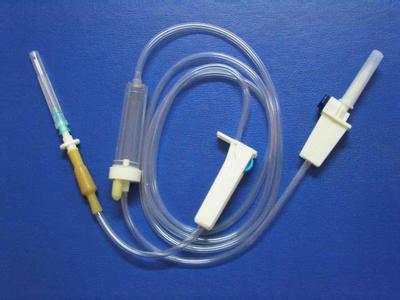
INFUSION SET with two plastic inserters two adjustors
Item No: YD180
This is disposable infusion set with two plastic inserters and two adjustors.
Assesoris: Vented spike, dripping champer, roller clamp, solution filter, emulsion tube.
Features:
1) For single use
2) Transparent drip chamber with filter
3) Flexible tubing with standard length of 135cm
4) Thick latex connector
5) Luer lock adapter with hypodermic needle
6) Sterilization: Ethylene-gas-sterilization
| Name of Sample | Disposable Infusion Set with needle | Type | IS-VZ  0.7 | ||||
| LOT NO | 20130618 | Testing Standards | contract requirements | ||||
| Sterilization Lot | 20130618 | Quantity of Tested Batch | 1260000 pcs | ||||
| Quantity Of Sample | 50pcs | Testing Date | SEP 7, 2013 | ||||
| Type | Testing items | Standard Requirement and Standard Term and Conditions | Actual Testing Result | Conclusion | |||
| Biological Properties | Sterility | The infusion set in individual package should be sterile (8.2) | Missing | / | |||
| Pyrogen | Infusion set has no pyrogen (8.3) | Missing | / | ||||
|  Chemical Properties |
Reduction Matter | Difference of volumes of potassium permanganate [ c(kmno4 = 0.002mol/L)] consumed by test liquid and blank liquid should be not more than 2.0 ml (7.1) | 0.5ml | Eligible | |||
| Ph | The PH difference between testing liquid and blank liquid is not more than 1.5 (7.3) | 0.19 | Eligible | ||||
| Ultraviolet Absorbance | The absorbance of testing liquid should be not more than 0.1 (7.5) | 0.046 | Eligible | ||||
| Ethylene Oxide Residuum | The ethylene oxide residuum should be not more than0.5mg; Unit: mg  (7.6) |    0.29 | / | ||||
|  Physical Properties |
Particle Content | For the number of 15-25min particles in 200ml eluent, not more than 1 particle/ml | Eligible | Eligible | |||
| For the number over 25min particles, not more than 0.5 particle/ml (6.1) | Eligible | ||||||
| Sealing Property | No air leakage of infusion set (6.2) | Eligible | Eligible | ||||
| Connection Intensity | Connection of parts for liquid channel of infusion set can bear the static tensile force not less than 15N for 15 seconds. (6.3) | Eligible | Eligible | ||||
| Puncture Device Of Bottle Stopper | According to the contract requirements ,Materials for ABS. |
Eligible | Eligible | ||||
| Liquid Medicine Fitter | The infusion set should be equipped with a liquid machine filter | Eligible | Eligible | ||||
| The filtering rate of liquid medicine should be not less than 80%. (6.7) | 98.7% | ||||||
| Flow Speed Of Transfusion (Flow Quantity) | For the infusion set with 20drops/ml, with 1m static press end, the sodium chloride solution flowed should be not less than 1000ml for 10 minutes Unit: ml  (6.10) |
Eligible | Eligible | ||||
| Air Inlet Device (Air Fitter) | The filtering rate against particles 0.5μm in air should be not less than 90%. (6.5.2) | Missing | / | ||||
| Air Inlet Device (Flow Reduction Rate ) | Comparing with vessel with free air input, the flow of effluent should be reduced by 20%. (6.5.5) | Eligible | Eligible | ||||
| Packaging requirements | According to the contract requirements | Eligible | Eligible | ||||
| Flow Adjustor | Materials for ABS. | Eligible | Eligible | ||||
| Soft Pipe (Length) | According to the contract requirements | Eligible | Eligible | ||||
| needle | According to the contract requirements | Eligible | Eligible | ||||
| Testing Results | The seized products meet the requirements of the  contract . |
||||||
INFUSION SET with two plastic inserters two adjustors
Item No: YD180
This is disposable infusion set with two plastic inserters and two adjustors.
Assesoris: Vented spike, dripping champer, roller clamp, solution filter, emulsion tube.
Features:
1) For single use
2) Transparent drip chamber with filter
3) Flexible tubing with standard length of 135cm
4) Thick latex connector
5) Luer lock adapter with hypodermic needle
6) Sterilization: Ethylene-gas-sterilization

| Name of Sample | Disposable Infusion Set with needle | Type | IS-VZ  0.7 | ||||
| LOT NO | 20130618 | Testing Standards | contract requirements | ||||
| Sterilization Lot | 20130618 | Quantity of Tested Batch | 1260000 pcs | ||||
| Quantity Of Sample | 50pcs | Testing Date | SEP 7, 2013 | ||||
| Type | Testing items | Standard Requirement and Standard Term and Conditions | Actual Testing Result | Conclusion | |||
| Biological Properties | Sterility | The infusion set in individual package should be sterile (8.2) | Missing | / | |||
| Pyrogen | Infusion set has no pyrogen (8.3) | Missing | / | ||||
|  Chemical Properties |
Reduction Matter | Difference of volumes of potassium permanganate [ c(kmno4 = 0.002mol/L)] consumed by test liquid and blank liquid should be not more than 2.0 ml (7.1) | 0.5ml | Eligible | |||
| Ph | The PH difference between testing liquid and blank liquid is not more than 1.5 (7.3) | 0.19 | Eligible | ||||
| Ultraviolet Absorbance | The absorbance of testing liquid should be not more than 0.1 (7.5) | 0.046 | Eligible | ||||
| Ethylene Oxide Residuum | The ethylene oxide residuum should be not more than0.5mg; Unit: mg  (7.6) |    0.29 | / | ||||
|  Physical Properties |
Particle Content | For the number of 15-25min particles in 200ml eluent, not more than 1 particle/ml | Eligible | Eligible | |||
| For the number over 25min particles, not more than 0.5 particle/ml (6.1) | Eligible | ||||||
| Sealing Property | No air leakage of infusion set (6.2) | Eligible | Eligible | ||||
| Connection Intensity | Connection of parts for liquid channel of infusion set can bear the static tensile force not less than 15N for 15 seconds. (6.3) | Eligible | Eligible | ||||
| Puncture Device Of Bottle Stopper | According to the contract requirements ,Materials for ABS. |
Eligible | Eligible | ||||
| Liquid Medicine Fitter | The infusion set should be equipped with a liquid machine filter | Eligible | Eligible | ||||
| The filtering rate of liquid medicine should be not less than 80%. (6.7) | 98.7% | ||||||
| Flow Speed Of Transfusion (Flow Quantity) | For the infusion set with 20drops/ml, with 1m static press end, the sodium chloride solution flowed should be not less than 1000ml for 10 minutes Unit: ml  (6.10) |
Eligible | Eligible | ||||
| Air Inlet Device (Air Fitter) | The filtering rate against particles 0.5μm in air should be not less than 90%. (6.5.2) | Missing | / | ||||
| Air Inlet Device (Flow Reduction Rate ) | Comparing with vessel with free air input, the flow of effluent should be reduced by 20%. (6.5.5) | Eligible | Eligible | ||||
| Packaging requirements | According to the contract requirements | Eligible | Eligible | ||||
| Flow Adjustor | Materials for ABS. | Eligible | Eligible | ||||
| Soft Pipe (Length) | According to the contract requirements | Eligible | Eligible | ||||
| needle | According to the contract requirements | Eligible | Eligible | ||||
| Testing Results | The seized products meet the requirements of the  contract . |
||||||
What is Cosmetic raw materials?
In the Arbas region of 105° east longitude and 40° north latitude where the temperature fluctuation between day and night can reach 50 degrees in winter, there is an essential native goat breed, the purebred Arbas Baby Cashmere Goat, which features thin and soft hair, a pair of horns and pink ears. Arbas Baby Cashmere Goat looks small yet are the nobles of goats.
The famous Arbas Baby Cashmere Goat, as a rare breed living in the Ordos Plateau of Inner Mongolia, is concentrated in Otog Banner, Otog Front Banner and Hanggin Banner in the western part of the Plateau. Sumu, Arbas, Otog Banner is the primary production area of Arbas Baby Cashmere Goat for its favorable natural environment, with Arbas Mountain in the west, vast natural pasture in the center and desert steppe in the south. The semi-arid steppe climate provides excellent conditions for animal husbandry.
Due to its location in the desert steppe, the area is dry and windy with little rainfall and much sand. The temperature difference between day and night is extremely high, and the annual average temperature is 6.4 °C. In the long, cold and dry winter, the lowest temperature reaches -30°C to -40°C late at night, while in the short, hot and arid summer, the highest temperature is 36.4°C and the lowest -32°C, with annual precipitation between 200mm to 400mm. The adorable, lovely and lively Arbas Baby Cashmere Goat grows in such extremely harsh conditions, thus can produce rare and quality cashmere.
Arbas Baby Cashmere Goat`s two coats are pure white. The top layer or outer coat is bright and coarse guard hair to protect the undercoat that is softer and finer down. The quality of the cashmere fleece is determined by three indicators: the diameter, length, and density of cashmere fibers. Due to its unique genes, Arbas Baby Cashmere Goat has a superiority that can never be duplicated. Its secondary hair follicle is smaller than those of other goats, and its cashmere has an average diameter between 13μm to 15 μm. It can even produce cashmere with a perfect diameter of 14.5μm, with a pure cashmere content of over 55%, which is unique worldwide. The preciousness of cashmere results from the small amount of production of each goat. Therefore, Inner Mongolian Arbas Baby Cashmere Goat was officially named by the People`s Government of Autonomous Region in 1988 and was listed on the National Register for the Conservation of Animal Genetic Resources as Class-1 protected breed by the Ministry of Agriculture in 2001.
Cosmetics Materials, cosmetics, Arbutin, Cosmetics raw Materials, cosmetics powder
Xi'an Day Natural Inc. , https://www.dayextract.com