Survival is over 3 years! Positive progress in brain cancer vaccine research
June 01, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Northwest Bio, which specializes in the development of individualized cancer vaccines, published the results of a phase 3 clinical trial showing that its dendritic cell vaccine improves the survival rate of patients with newly diagnosed glioblastoma, and some patients survive. The period has exceeded the standard treatment survival time by twice or even nearly three times. Related data is published in the Journal of Translational Medicine.

Glioblastoma is a tumor produced by glial cells or their precursors in the central nervous system (CNS), which is the most invasive and most common CNS malignancy, accounting for 47% of such tumors. According to statistics, there were approximately 12,000 new glioblastomas in the United States in 2017. In 2015, there were approximately 101,600 new cases of brain cancer or CNS cancer in China. The median survival of this type of cancer is about 15 months, and the five-year survival rate is only 5.5%. Due to the heterogeneity of the blood-brain barrier and tumor cells, such cancers are extremely difficult to treat. Survival rates are still very low even for patients undergoing surgery and high doses of chemotherapy and radiation. For patients with recurrent glioblastoma, treatment options are even more limited. These patients urgently need effective therapies to prolong life.
Northwest Bio's cancer vaccine has achieved positive results in the treatment of glioblastoma. Its DCVax platform is designed to create individualized therapies for a variety of biomarkers in patient tumors. It draws blood from the patient, extracts monocytes, differentiates them into dendritic cells, and then "activates" the antigen combination from the patient's tumor. The thus treated cells are then injected back into the patient to identify and attack the cancer.
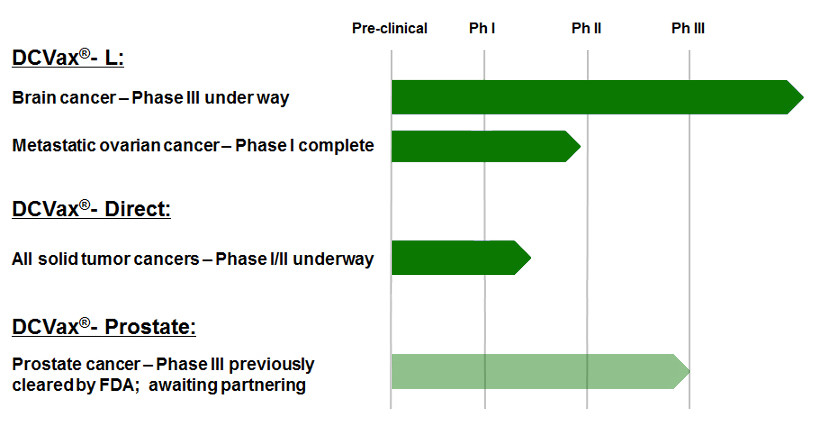
â–²Northwest Bio research product line (Source: Northwest Bio official website)
The data presented this time was from a double-blind, placebo-controlled phase 3 clinical trial. The 331 patients enrolled in the trial were randomized, and 2/3 of the patients received standard treatment (ie, chemotherapy and radiotherapy after surgical removal of the tumor), plus Northwest Bio's vaccine DCVax-L, and the remaining 1/3 of patients received standard treatment plus Placebo. But in the end, about 90% of patients have received DCVax-L because those with tumor recurrence may “span†into the treatment group and receive the vaccine, blindly before in the vaccine or placebo group. Therefore, the results were counted in patients with both the vaccine and placebo.
The results showed that patients with both arms had a median survival of 23.1 months after surgery, and 34.7 months for patients with methylated MGMT gene. The MGMT gene predicts patients' sensitivity to alkylation chemotherapy (such as temozolomide). Signs. The median survival time of patients with unmethylated MGMT was 19.8 months, still higher than the median survival of standard treatment for 15 to 16 months. At the time of statistical results, 223 patients had a postoperative survival of more than 30 months, and 100 patients had a postoperative survival of 40.5 months. But Northwest Bio has not yet determined the reasons for this positive result. Those known to have good prognostic factors, such as age less than 50 years, methylated MGMT gene and surgery to completely remove all tumors, etc., only 8% of these 100 patients were in good condition among all of these prognostic factors. The data also shows that once patients have survived for more than a certain period of time, they are likely to "continue to survive for a considerable period of time." The incidence of serious adverse events was 2% in this trial, and the overall incidence of adverse events was comparable to standard treatment.

â–²Ms. Linda Powers, CEO of Northwest Bio (Source: Northwest Bio Official Website)
“These are just temporary data, and as the data matures, the results may vary,†said Linda Powers, CEO of Northwest Bio, in a statement: “But there has been limited progress in the treatment of glioblastoma for decades. In the case, we saw an extended survival time, which is encouraging."
We expect this personalized vaccine to bring good news to patients with glioblastoma!
Reference materials:
[1] Northwest Bio's long-awaited glioblastoma vaccine data see stock roller coaster
[2] Northwest Bio official website
Reflective Sheeting,3M Reflective Sheeting,Best Reflective Tape,Colored Reflective Tape
Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.yuhuanptapes.com